A newly biocompatible, anti-thrombogenic vascular membrane
Cardiovascular diseases have been the leading cause of mortality, according to the World Health Organization (WHO) for the past few years. In 2021, specifically in Malaysia the Department of Statistics recorded that 17% of Malaysians died due to ischemic heart disease as the topmost ranking, Followed by pneumonia (11.4%), cerebrovascular diseases (8.3%) and transport accidents (2.9%).
Most cardiovascular diseases require vascular reconstructive surgery to remove affected tissue lesions. Several vascular membranes/grafts are commercially available to replace defective tissues in vascular reconstructive surgery. However, synthetic commercialized vascular membranes/grafts known as GORE-TEX® (made of polytetrafluoroethylene (PTFE)) and Dacron® (made of polyethene terephthalate (PET)) are not biodegradable.
They are reported with post-complication of thrombotic formation following the implantation procedure. Although myriad tissue engineering approaches have been considered to fabricate biodegradable vascular membranes/grafts to enhance biocompatibility, extensive investigation is still required to develop new generation vascular membranes/grafts that can match the compliance of native vascular tissues.
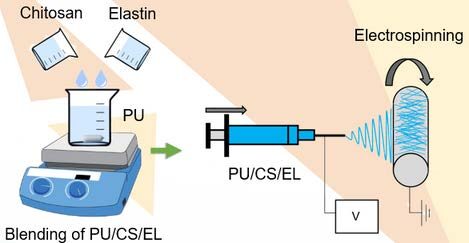
In our team’s research exploration, biodegradable polyurethane (PU) has been blended with elastin and chitosan nanoparticles as an initiative of vascular membrane development to overcome the limitations of non-biodegradable and thrombus generation following vascular surgery. This formulation is called ChitoE, which uses electrospinning to form polymeric mesh nanofibers.
Electrospinning can create fibres with diameters ranging from micro to nanometer with a structure that resembles the extracellular matrix (ECM) of native small-diameter blood vessels. The structural electrospun elastomeric fibre networks allow the scaffolds to possess high surface-to-volume ratios and porosities that expedite cell attachment and ingrowth.
It involves the application of an electric field on the polymer solution to produce nanofibrous polymer scaffolds. The device setup includes a syringe consisting of polymer solution mounted on the syringe pump and connected with the high applied voltage. The ejected nanofibrous material can be collected using a rotating collector to create tubular scaffolds or a flat collector to form thin film membranes.
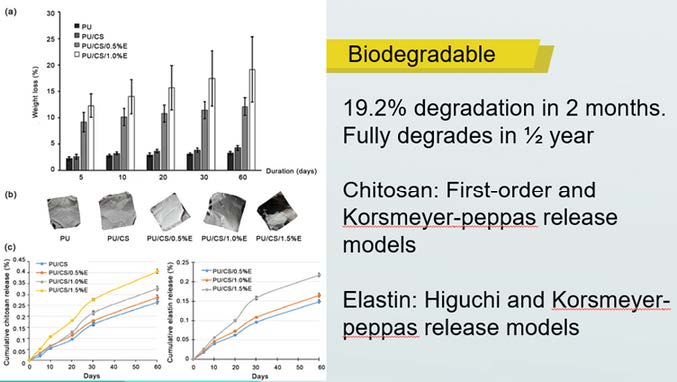
This project collaborates with the National Heart Institute (IJN Sdn. Bhd.) to respond to the current clinical complication of vascular membranes and grafts at IJN. Dr. Sivakumar Sivalingam, the IJN cardiothoracic surgeon, who is a part of the team members, actively participated in lending clinical guidance during the development progress of the ChitoE product. The ChitoE is currently at TRL 4 for the in-vivo implantation for its physiological verification. It has been proven biodegradable, biocompatible, anti-thrombogenic and thermally stable.