July 2024 Research Ethics Application Is Now Opened
Application Opening Date
1st July 2024
Application Deadline
29th July 2024
UTM REC 20th Main Panel Meeting
29 August 2024
UTM Research Ethics Committee
Overview
The Universiti Teknologi Malaysia Research Ethics Committee (UTM REC) conducts an ethics review prior to the beginning of any research involving human and animals. This committee is empowered to evaluate ethical compliance of research protocols based on available international ethical and scientific quality standards. The UTM REC structure has met the guidelines of International Conference on Harmonization-Good Clinical Practice (ICH-GCP)
Objectives
To ensure quality and consistency in the review of research protocols
Quality & Consistency
To ensure that the research is conducted in a way that serves the interests of individuals or society as a whole
Interests of Society
To ensure that the research is followed the international ethical guidelines for clinical, non-clinical and animal research
Compliance With Guidelines
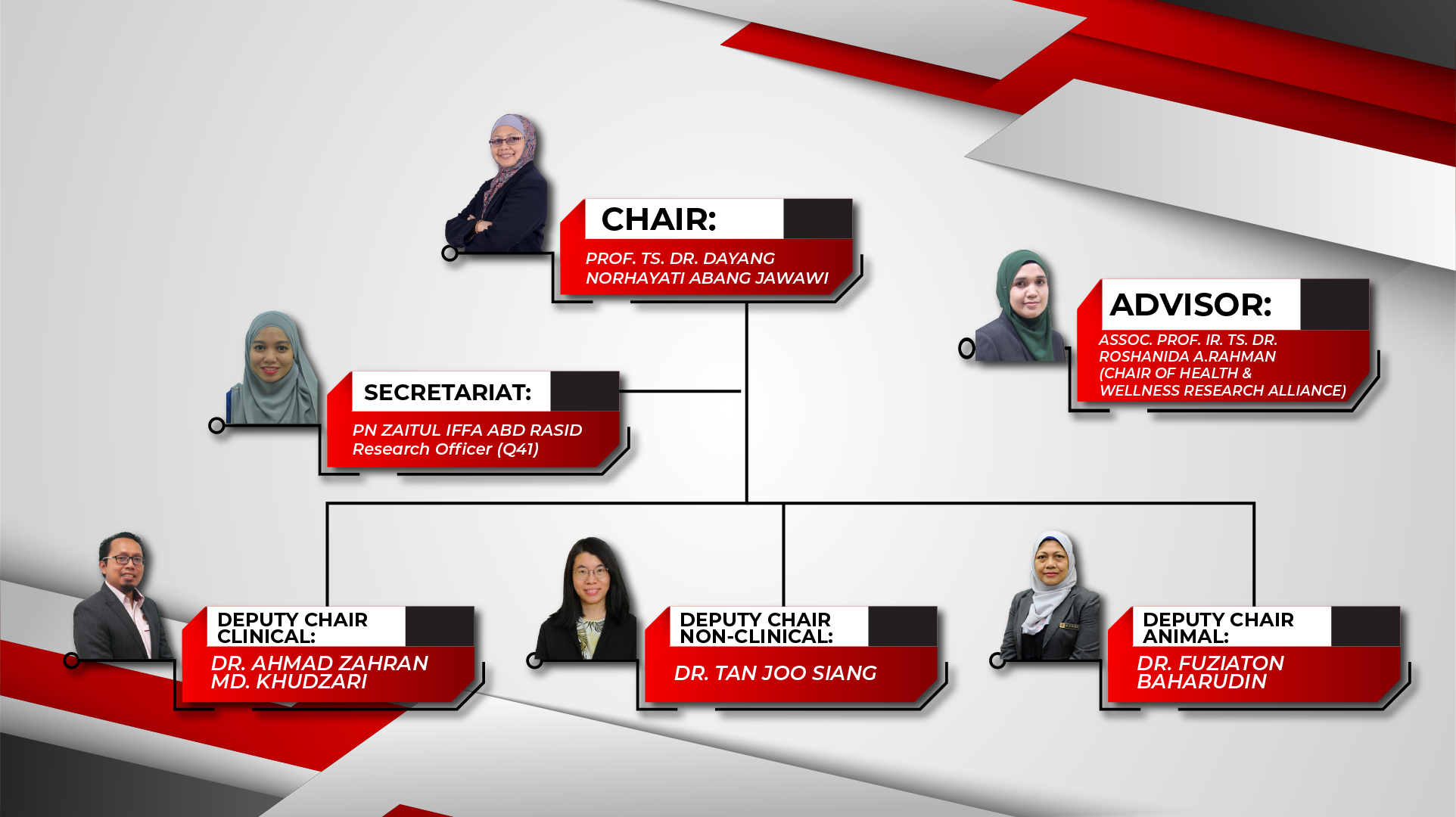
Main Structure of UTM REC
Application & Guidelines
Other REC Forms
- UTM REC Application Forms
- Clinical Ethics
- Non-Clinical Ethics
- Animal Ethics
- UTM REC Application Checklists
- Clinical Ethics and Non-Clinical Ethics
- Animal Ethics
- Continuing Review Form
- Revision of Research Form
- Amendment of Research Form
- Extension of Research Form
- Extension of Resarch Ethics Application Submission
- Justification of Withdrawal Form
Application Form
All the application forms should be computer typed. Handwritten forms will be rejected.
Application Fee: RM 150 (non-refundable)
Payment direct to account:
Bendahari UTM
CIMB Bank Berhad
Account No: 8006053536
Reference: Ethics Application
Contact Us
UTM Research Ethics Committee
Office of Deputy Vice Chancellor (Research and Innovation)
Universiti Teknologi Malaysia,
81310, Johor Bahru Johor.
Email: ethics@utm.my
Meeting & Approval
The meetings will be held once a month and the approval emails will be sent to applicants via official email ethics@utm.my.
UTM REC will take about 30 days to process your application after the submission deadline.
However, the processing time might take up to 60 days depending on the research complexity. Please keep in mind that required amendments will require additional processing time.
Applicants are advised to submit their ethics clearance application as EARLY as possible prior to their data collection.
2024 Schedules
| No. | Application Month | Application Opening Date | Application Deadline | UTM REC Main Panel Meeting |
|---|---|---|---|---|
| 1 | January | 1 January 2024 | 30 January 2024 | 28 February 2024 |
| 2 | February | 1 February 2024 | 27 February 2024 | 3 April 2024 |
| 3 | March | 1 March 2024 | 28 March 2024 | 5 May 2024 |
| 4 | April | 1 April 2024 | 27 April 2024 | 9 June 2024 |
| 5 | May | 1 May 2024 | 29 May 2024 | 27 June 2024 |
| 6 | June | 1 June 2024 | 27 June 2024 | 30 July 2024 |
| 7 | July | 1 July 2024 | 29 July 2024 | 29 August 2024 |
| 8 | August | 1 August 2024 | 29 August 2024 | 26 September 2024 |
| 9 | September | 1 September 2024 | 27 September 2024 | 29 October 2024 |
| 10 | October | 1 October 2024 | 29 October 2024 | 26 November 2024 |
| 11 | November | 1 November 2024 | 28 November 2024 | 31 December 2024 |
| 12 | December | 1 December 2024 | 27 December 2024 | 28 January 2025 |
*The date is subject to change by UTM REC
Approval Status
| No. | Application No. | Current Status | Approval No. | Date of Approval |
|---|---|---|---|---|
| 1 | NC-11-22-01 | Approved | UTMREC-2023-02 | 26 January 2023 |
| 2 | NC-11-22-02 | Approved | UTMREC-2023-03 | 26 January 2023 |
| 3 | NC-11-22-03 | Approved | UTMREC-2023-04 | 26 January 2023 |
| 4 | NC-11-22-04 | Approved | UTMREC-2023-01 | 18 December 2022 |
| 5 | A-11-22-05 | Rejected | - | - |
| 6 | C-11-22-06 | Approved | UTMREC-2023-25 | 25 June 2023 |
| 7 | NC-11-22-07 | Rejected | - | - |
| 8 | NC-11-22-08 | Approved | UTMREC-2023-05 | 26 January 2023 |
| 9 | NC-11-22-09 | Approved | UTMREC-2023-10 | 23 February 2023 |
| 10 | C-11-22-10 | Approved | UTMREC-2023-14 | 2 April 2023 |
| 11 | NC-11-22-11 | Approved | UTMREC-2023-11 | 23 February 2023 |
| 12 | NC-11-22-12 | Rejected | - | - |
| 13 | A-11-22-13 | Approved | UTMREC-2023-06 | 26 January 2023 |
| 14 | C-11-22-14 | Approved | UTMREC-2023-11 | 23 February 2023 |
| 15 | A-12-22-15 | Revise | ||
| 16 | A-12-22-16 | Approved | UTMREC-2023-07 | 26 January 2023 |
| 17 | A-12-22-17 | Withdraw | - | - |
| 18 | NC-12-22-18 | Approved | UTMREC-2023-12 | 23 February 2023 |
| 19 | NC-12-22-19 | Approved | UTMREC-2023-08 | 26 January 2023 |
| 20 | NC-12-22-20 | Approved | UTMREC-2023-09 | 26 January 2023 |
| 21 | NC-01-23-21 | Approved | UTMREC-2023-13 | 23 February 2023 |
| 22 | NC-01-23-22 | Approved | UTMREC-2023-16 | 2 May 2023 |
| 23 | NC-01-23-23 | Approved | UTMREC-2023-15 | 2 April 2023 |
| 24 | NC-01-23-24 | Rejected | - | - |
| 25 | NC-02-23-25 | Approved | UTMREC-2023-20 | 6 Jun 2023 |
| 26 | NC-02-23-26 | Approved | UTMREC-2023-17 | 2 May 2023 |
| 27 | NC-02-23-27 | Approved | UTMREC-2023-21 | 6 Jun 2023 |
| 28 | NC-02-23-28 | Approved | UTMREC-2023-18 | 2 May 2023 |
| 29 | C-02-23-29 | Approved | UTMREC-2023-22 | 6 Jun 2023 |
| 30 | A-03-23-30 | Approved | UTMREC-2023-26 | 25 June 2023 |
| 31 | A-03-23-31 | Withdraw | - | - |
| 32 | NC-03-23-32 | Approved | UTMREC-2023-23 | 6 Jun 2023 |
| 33 | C-03-23-33 | Withdraw | - | - |
| 34 | NC-03-23-34 | Approved | UTMREC-2023-24 | 6 Jun 2023 |
| 35 | A-04-23-35 | Withdraw | - | - |
| 36 | A-04-23-36 | Approved | UTMREC-2023-29 | 7 July 2023 |
| 37 | NC-04-23-37 | Approved | UTMREC-2023-28 | 7 July 2023 |
| 38 | NC-04-23-38 | Approved | UTMREC-2023-19 | 31 May 2023 |
| 39 | C-04-23-39 | Approved | UTMREC-2023-27 | 25 June 2023 |
| 40 | C-05-23-40 | Approved | UTMREC-2023-34 | 1 November 2023 |
| 41 | NC-06-23-41 | Approved | UTMREC-2023-30 | 1 October 2023 |
| 42 | NC-06-23-42 | Revise | ||
| 43 | C-07-23-43 | Approved | UTMREC-2023-33 | 1 November 2023 |
| 44 | NC-07-23-44 | Approved | UTMREC-2023-32 | 1 November 2023 |
| 45 | NC-07-23-45 | Approved | UTMREC-2023-31 | 1 October 2023 |
| 46 | NC-07-23-46 | Approved | UTMREC-2023-35 | 1 November 2023 |
| 47 | NC-07-23-47 | Approved | UTMREC-2023-37 | 4 December 2023 |
| 48 | NC-08-23-48 | Approved | UTMREC-2023-39 | 26 December 2023 |
| 49 | NC-08-23-49 | Approved | UTMREC-2023-40 | 26 December 2023 |
| 50 | NC-08-23-50 | Approved | UTMREC-2023-41 | 26 December 2023 |
| 51 | NC-08-23-51 | Approved | UTMREC-2024-46 | 1 February 2024 |
| 52 | NC-08-23-52 | Approved | UTMREC-2023-40 | 26 December 2023 |
| 53 | C-08-23-53 | Approved | UTMREC-2023-36 | 15 November 2023 |
| 54 | NC-09-23-54 | Approved | UTMREC-2024-47 | 1 February 2024 |
| 55 | NC-09-23-55 | Approved | UTMREC-2023-38 | 4 December 2023 |
| 56 | C-10-23-56 | Approved | UTMREC-2023-42 | 26 December 2023 |
| 57 | NC-10-23-57 | Approved | UTMREC-2024-49 | 28 February 2024 |
| 58 | NC-10-23-58 | Approved | UTMREC-2023-43 | 26 December 2023 |
| 59 | NC-10-23-59 | Approved | UTMREC-2023-44 | 26 December 2023 |
| 60 | A-11-23-60 | Approved | UTMREC-2024-51 | 1 February 2024 |
| 61 | NC-11-23-61 | Approved | UTMREC-2024-49 | 1 February 2024 |
| 62 | NC-11-23-62 | Approved | UTMREC-2024-53 | 29 February 2024 |
| 63 | NC-11-23-63 | Approved | UTMREC-2023-45 | 26 December 2023 |
| 64 | C-12-23-64 | Approved | UTMREC-2023-E1 | 12 December 2023 |
| 65 | NC-12-23-65 | Approved | UTMREC-2024-55 | 3 April 2024 |
| 66 | C-12-23-66 | Approved | UTMREC-2024-56 | 3 April 2024 |
| 67 | A-12-23-67 | Approved | UTMREC-2024-52 | 14 February 2024 |
| 68 | NC-12-23-68 | Revise | ||
| 69 | NC-12-23-69 | Revise | ||
| 70 | NC-12-23-70 | Approved | UTMREC-2024-67 | 7 May 2024 |
| 71 | NC-12-23-71 | Approved | UTMREC-2024-53 | 29 February 2024 |
| 72 | A-12-23-72 | Approved | UTMREC-2024-50 | 4 February 2024 |
| 73 | C-01-24-73 | Approved | UTMREC-2024-65 | 7 May 2024 |
| 74 | C-01-24-74 | Approved | UTMREC-2024-57 | 3 April 2024 |
| 75 | NC-01-24-75 | Approved | UTMREC-2024-58 | 3 April 2024 |
| 76 | NC-01-24-76 | Approved | UTMREC-2024-59 | 3 April 2024 |
| 77 | NC-01-24-77 | Approved | UTMREC-2024-68 | 9 June 2024 |
| 78 | NC-01-24-78 | Rejected | - | - |
| 79 | C-02-24-79 | Withdraw | - | - |
| 80 | NC-02-24-80 | Withdraw | - | - |
| 81 | NC-02-24-81 | Approved | UTMREC-2024-60 | 3 April 2024 |
| 82 | NC-02-24-82 | Approved | UTMREC-2024-63 | 7 May 2024 |
| 83 | NC-02-24-83 | Approved | UTMREC-2024-64 | 7 May 2024 |
| 84 | NC-02-24-84 | Approved | UTMREC-2024-61 | 3 April 2024 |
| 85 | NC-02-24-85 | Approved | UTMREC-2024-62 | 3 April 2024 |
| 86 | NC-03-24-86 | Approved | UTMREC-2024- 69 | 9 June 2024 |
| 87 | C-03-24-87 | Approved | UTMREC-2024-70 | 9 June 2024 |
| 88 | C-03-24-88 | Approved | UTMREC-2024-71 | 9 June 2024 |
| 89 | NC-03-24-89 | Revise | ||
| 90 | NC-03-24-90 | Approved | UTMREC-2024-72 | 9 June 2024 |
| 91 | NC-03-24-91 | Revise | ||
| 92 | NC-03-24-92 | Approved | UTMREC-2024-66 | 7 May 2024 |
| 93 | NC-04-24-93 | Revise | ||
| 94 | NC-04-24-94 | Revise | ||
| 95 | NC-04-24-95 | Approved | UTMREC-2024-73 | 9 June 2024 |
| 96 | NC-04-24-96 | Revise | ||
| 97 | NC-05-24-97 | Revise | ||
| 98 | NC-05-24-98 | Approved | ||
| 99 | NC-05-24-99 | Approved | ||
| 100 | NC-05-24-100 | Revise | ||
| 101 | NC-06-24-101 | New Submission | ||
| 102 | NC-06-24-102 | New Submission | ||
| 103 | NC-06-24-103 | New Submission | ||
| 104 | NC-06-24-104 | New Submission |