
Chemical-Looping – An Environmentally Friendly Approach for Energy and Chemicals
Mohammad M. Hossain
Department of Chemical Engineering, Interdisciplinary Research Center for Refining & Advanced Chemicals (IRC-RAC),
King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia
Abstract – In the last two decades, significant progress has been made towards a better understanding of world climate change, its long-term impact and searching solutions for the sustainable environment. In this regard, chemical looping combustion (CLC) has been extensively investigated as a promising approach for CO2 capture from fossil fuel-based power plants. A Circulating fluidized bed process involving a durable oxygen carrier can open the door for CLC with inherent CO2 capture using NG, diesel, heavy residues, coal and biomass as fuels in industrial scale electricity generation. Therefore, the application of CLC and CO2 capture/use will secure the continuation of cheaper fossil energy-based power generation until the renewable alternatives reach to their competitiveness. On the other hand, the chemical looping approach can also be implemented for chemical and hydrogen production – which will not only enhance the overall process efficiency but also significantly contribute to the minimization of CO2 emission. The chemical-looping strategy offers opportunities for process intensification and exergy loss minimization. An integrated CLC and chemical-looping chemical/hydrogen production will also create the opportunity for capture and use of CO2 in chemicals production – a way of realization of the circular carbon economy. In many cases, the reduction of energy consumption is significant enough that the decrease of CO2 emission target can be achieved without CO2 capture. The present keynote will report the work in progress on both the CLC and Chemical-looping chemical/hydrogen production at Chemical Engineering-KFUPM.
Converting Plants Waste into Solvent as Green Approaches for Synthesis of Nanoparticles Photocatalyst
Nur Farhana Jaafar
School of Chemical Sciences, Universiti Sains Malaysia,
11800 USM Penang, Penang, Malaysia
E-mail: nurfarhana@usm.my
Abstract – Wastewater treatment of persistent organic pollutants (POPs) faces several key challenges like high cost, large energy consumption and emerging contaminants. Photocatalysis is an advanced oxidation process (AOPs), which is a promising technology for treating wastewater contaminated with POPs in recent years with the aid of photocatalyst. Nowadays, various methods of synthesis have been explored and modified to produce photocatalysts with suitable properties where most of the methods used a lot of chemicals and consequently contributed to formation of more waste. Hence, to reduce the use of chemicals by prioritizing the green and sustainable aspects, exploring natural and green resources in photocatalyst synthesis is one of the alternatives. This work presents the potential of various sources of plant wastes and turns into extract to be used as alternative solvents for synthesis of various nanoparticles photocatalysts. Plants waste extract (PWE) containing rich amounts of useful bioactive components (phenolic, myricetin, flavanols) play an important role in assisting the production of nanoparticles. It has been known that active biocomponents from plant resources are employed to serve as the reducing, stabilizing, or capping agent during material growth. The results revealed that the reducing capacity of PWE during material synthesis was linked to their total phenolic and flavonoid content as well as the plant-based wastes extract with higher phenolic content generated smaller particle sizes and surface oxygen vacancies of photocatalyst, which contributed to enhanced pollutant degradation performance. Improved properties of photocatalyst give more potential for their ability to degrade various pollutants.
Keywords: plant waste; photocatalyst; photodegradation; wastewater
PdZn/ZnO−TiO2 catalysts for CO2 hydrogenation to methanol
Athirah Ayuba, Abdul Hanif Mahadia, Mohammd Ammar Syaahiran Alima, Hasliza Bahrujia*
aCentre for Advanced Material and Energy Sciences, Universiti Brunei Darussalam, Jalan Tungku Link, BE1410, Brunei Darussalam
*Corresponding author: hasliza.bahruji@ubd.edu.bn
Abstract – Carbon dioxide in the atmosphere has reached an alarming level and, therefore, requires a global effort to reduce emissions. CO2 hydrogenation to methanol (CTM) is one of the CCU technologies offering easy storage and transportation of liquid methanol [1]. CTM is an exothermic reaction releasing -49.5 kJmol-1 of energy at 298 K (Eqn. (1)). The reaction thermodynamically favored low temperature and high pressure conditions [2]. Porous ZnO/TiO2 synthesized using different Zn and Ti ratios (Zn:Ti = 0.5, 1, 2) is investigated as PdZn alloy support for CO2 hydrogenation to methanol. The partial transformation of ZnO/TiO2 into ZnTiO3 perovskite occurs on the PdZn perimeter, forming strong interfacial interaction with PdZn alloy. Variation of Zn:Ti ratios affected the formation of cubic and hexagonal ZnTiO3. At low Zn:Ti ratio of 0.5, the cubic ZnTiO3 generates a high density of surface oxygen vacancies, achieving ~1121.3 mmolkg-1h-1 methanol after 24 h reaction. The trace amount of methane and C2+ hydrocarbons from the C−C coupling reaction occur at 450°C. In-situ DRIFTS analysis provides insight into the reaction mechanism that occurs via two intermediate pathways i.e., formate and formyl *HCO species.
Keywords: PdZn alloy, CO2 hydrogenation, methanol, ZnTiO3 interface
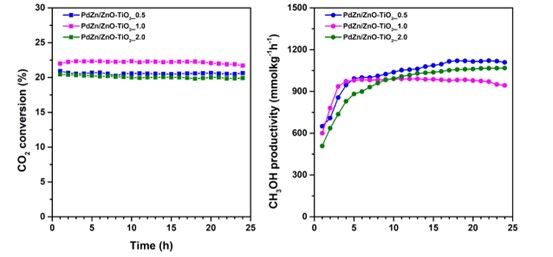
Fig 1. (a) CO2 conversion on PdZn/ZnO−TiO2 catalysts at different Zn:Ti ratios (0.5. 1.0 and 2.0) at reaction temperature 300°C and pressure 20 bar for 24 h and (b) methanol productivity.
References:
- D. Masih, S. Rohani, J. N. Kondo, and T. Tatsumi, “Low-temperature methanol dehydration to dimethyl ether over various small-pore zeolites,” Applied Catalysis B: Environmental, vol. 217, pp. 247–255, Nov. 2017, doi: 10.1016/j.apcatb.2017.05.089.
- W. Wang, S. Wang, X. Ma, and J. Gong, “Recent advances in catalytic hydrogenation of carbon dioxide,” Chem. Soc. Rev., vol. 40, no. 7, p. 3703, 2011, doi: 10.1039/c1cs15008a.

